
Principals


Perfuzia Medical
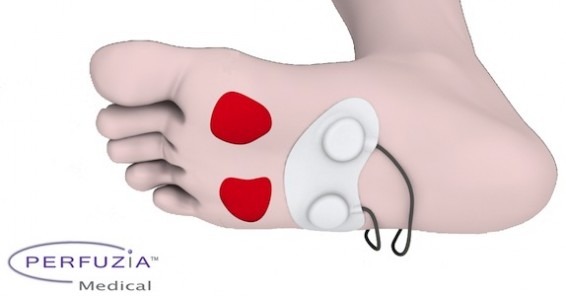
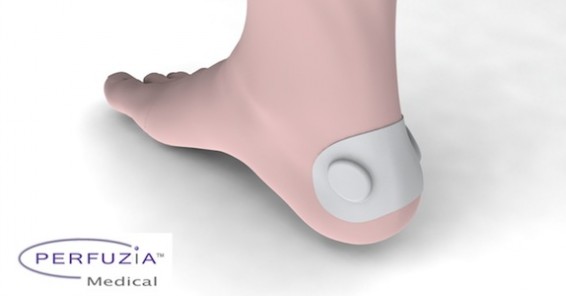
Perfuzia is a clinical-stage medical device company addressing a major unmet need in the $5B advanced wound healing market. They help rehabilitation and nursing facilities a novel, cost-effective product / technology to heal wounds faster while reducing costs and minimizing burden on over-taxed care giver staff. Perfuzia is pioneering the therapeutic use of local mechanical skin stimulation. The ActiveFlow ultra-portable adjunctive therapy device addresses the most critical drivers of successful wound healing – local perfusion (blood flow), oxygenation and woundbed metabolism. ActiveFlow has demonstrated statistically-significant proof-of-concept in healthy volunteers and very exciting early clinical results in chronic patients; They are now focused on clinical development in preparation for regulatory approvals and commercialization. Ideas and proposals for clinical-research collaborations are welcome, with emphasis on burns, venous ulcers, diabetic foot ulcers and bedsores, as well as orthopedics and sports medicine.
ActiveFlow is NOT approved for sale anywhere in the world at this time, including the US.
